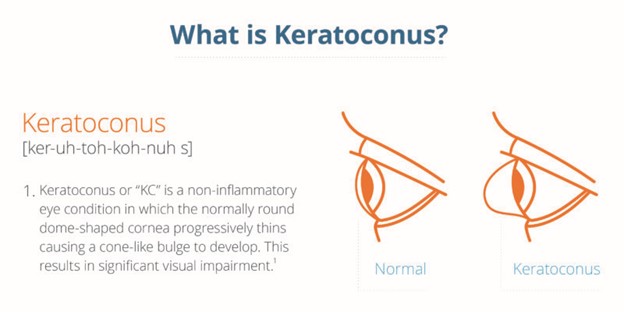
Keratoconus is an eye disorder that results in thinning and bulging of the cornea, which is a type of corneal ectasia. The usual round shape of the front part of the eye changes into a cone shape in patients with keratoconus due to pressure from within the eye that pushes out through the thin area.
This disease affects both eyes in a progressive manner, but sometimes, it may be asymmetrical. Symptoms often begin to manifest in late adolescence or early adulthood, but they can go unnoticed or undiagnosed for an extended period.
The cornea is commonly known as the “eye’s window,” as its shape directs light into the eye. As keratoconus changes the cornea’s shape, affected individuals become increasingly nearsighted and may develop sensitivity to light. The cornea also becomes more irregular, leading to progressively deteriorating vision that may be challenging to correct using glasses or contacts. If left untreated, this condition can significantly impact vision. The corneal bulge may worsen, and in severe cases, a corneal transplant may be the only viable treatment option available.
It is important to note that the goal of treatment for corneal cross-linking patients is to slow or halt the progression of the disease. For these patients, continued progression often results in loss of visual acuity or decreased tolerance to contact lens wear, caused by the ongoing changes in the cornea. Therefore, the earlier progressive keratoconus is diagnosed, the sooner treatment can be provided that may slow the progression of the disease.
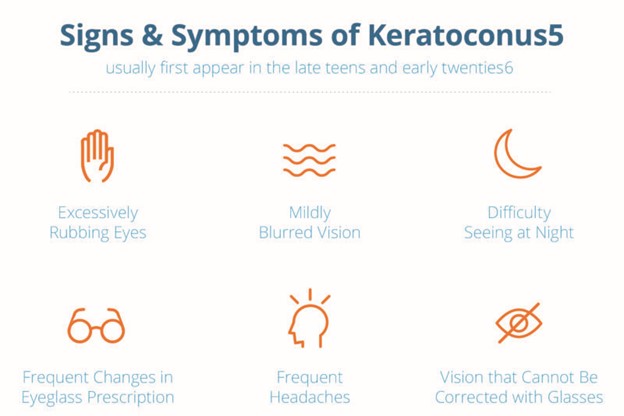
Early signs of keratoconus may include asymmetric refractive error, high or progressive astigmatism, or reduced best corrected visual acuity. The onset of keratoconus often occurs in teenage years or early twenties but can start at any time.
Patient symptoms may include:
Keratoconus, especially in the early stages, can be difficult to diagnose and
All of the above symptoms could be associated with other eye problems.
The iLink™ corneal cross-linking procedure is a non-invasive outpatient treatment that employs a combination of ultraviolet light and specially-formulated eye drops to strengthen and stiffen corneas weakened by disease or refractive surgery. This innovative technique has been widely accepted as the global standard of care for progressive keratoconus and corneal ectasia following refractive surgery.
The iLink™ corneal cross-linking procedure uses ultraviolet light to activate riboflavin eye drops that have been applied to the eye’s surface. This process results in the formation of chemical bonds between collagen fibers in the cornea, which strengthen and stiffen the tissue. The procedure is performed on an outpatient basis, and most patients can resume their normal activities within a few days.
In conclusion, the iLink™ corneal cross-linking procedure is a minimally-invasive outpatient treatment that provides patients with an innovative solution for treating keratoconus and corneal ectasia following refractive surgery. This procedure has been widely accepted as the global standard of care and has helped many patients regain their vision and quality of life. If you or a loved one is suffering from these conditions, it is worth exploring this innovative treatment option with your eye care professional.
Corneal crosslinking is a groundbreaking procedure that strengthens the cornea by adding bonds between bundles of collagen fibers. This process involves the use of a specific wavelength of UV light combined with riboflavin, also known as vitamin B2. The resulting change in the cornea’s fibers stabilizes weakened areas and makes it more rigid. Keratoconus, a disease that causes the cornea to bulge and distort, is the only condition that this treatment has been shown to halt or slow down.
Dr. Farhat uses only FDA-approved techniques and products to ensure patient safety. The procedure is performed in Heartland Eye Care and typically takes one to one and a half hours. The process is usually done one eye at a time.
The procedure begins with the administration of numbing drops and medication to keep the patient calm and comfortable. Once the eye is numb, the cornea’s epithelial surface is painlessly removed. Riboflavin drops are then administered every 2 minutes for about 30 minutes. The riboflavin penetrates the corneal tissues during this time.
Next, the patient will lie back and look at a bright light while a small, comfortable device keeps their eyelids open to prevent blinking during the procedure. The Avedro KXL UV lamp, the only FDA-approved technology for this use, targets a specific amount of UV light onto the cornea.
After the UV light exposure, a special contact lens is placed in the eye to protect it during the healing process. Patients receive a prescription for anti-inflammatory and topical antibiotic eye drops.
The crosslinking procedure is comfortable due to the numbing drops, and the staff will talk the patient through each step to ease any anxiety. After a brief evaluation and instruction, the patient can leave with a designated driver.

Under the conditions used for iLink™ corneal cross-linking, specially formulated pharmaceutical strength riboflavin eye drops called Photrexa® (riboflavin 5’-phosphate ophthalmic solution) and Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) help enable the cross-linking reaction.
iLink™ corneal cross-linking applies an artificial source of ultraviolet light from a machine called the KXL System once the cornea has been soaked in the Photrexa® and Photrexa® Viscous eye drops. This process works to stiffen the cornea by increasing the number of molecular bonds, or cross-links, in the collagen.
Using Photrexa® and Photrexa® Viscous riboflavin eye drops, combined with ultraviolet light from the KXL system, the iLink™ procedure stiffens and strengthens the cornea to slow or halt progressive keratoconus.
Yes, your doctor will apply topical anesthesia to numb the eye prior to the removal of the epithelium. This process helps prepare your eye so that the drug can penetrate the tissue of the cornea to have an effective iLink™ procedure. Today, this is the only cross-linking procedure deemed safe and effective by the United States FDA.
Yes, typically you will be awake during the treatment. You may be given relaxing medication and numbing anesthetic drops.
There is some discomfort during immediate recovery but usually not during the treatment. Immediately following treatment, a bandage contact lens is placed on the surface of the eye to protect the newly treated area. After the numbing drops wear off, there is some discomfort, often described as a gritty, burning sensation managed with acetaminophen and artificial tears. If pain is severe, oral narcotic medications may be prescribed.
No. There is no change in the appearance of your eyes following an iLink™ procedure.
Patients who have been diagnosed with progressive keratoconus should ask their doctor whether they may be an appropriate candidate for iLink™ corneal cross-linking.
Yes. Typically, doctors ask their patients to stop wearing contact lenses prior to surgery for several weeks. Once treated, patients may not be allowed back into contact lenses for up to 1 month.
The iLink™ procedure is widely covered by commercial insurance policies in the United States. Please contact your insurance carrier or your healthcare provider to understand any out-of-pocket costs you may be responsible for.
Please feel free to schedule an appointment with us to discuss your specific needs and to hear more about how this revolutionary technology can benefit you. We’d love to hear from you.
Heartland Eye Care & Washburn Surgery Center, Where Clear Vision Is Our Mission.
